A replication of part of Clive Best’s analysis.
The theory of catastrophic anthropogenic global warming assumes a number of points without evidence, assumptions that might well be true, but which they have made no attempt to test.
One is that warming would be a bad thing, another is that the world is very sensitive to quite small CO2 greenhouse effect, supposedly because the CO2 greenhouse effect will be enormously amplified by the H2O green house effect.
And another is that human burning of coal and oil can have a significant effect on atmospheric CO2. The latter point seems reasonable, since the amount of coal and oil that has been burnt is comparable to the amount of CO2 in the air. It is, however, insignificant compared to the amount of CO2 in the ocean, and the ground. The threat of acidifying the oceans with CO2 is incompatible with the threat that human are capable of significantly affecting the amount of CO2 in the air. If CO2 in the air rapidly equilibriates with CO2 in the oceans, then for humans to make a significant difference by burning coal would be like humans raising the level of lake Michigan by spitting in it. There is a lot of CO2 in the oceans. It is physically impossible for humans to change the acidity of any substantial part of the oceans.
CO2 in the air has been rising, at about half the rate that would be expected if all the CO2 in the coal we are burning stayed in the air, but, over the last twenty thousand years, CO2 has been a lot higher than it is now, and a lot lower than it is now, so the recent rise may well reflect forces far mightier than puny humankind. Usually rise in CO2 follows rise in global temperatures by a couple of hundred years, so we would expect CO2 levels to be naturally rising today, because we are recovering from the little ice age that ended during the nineteenth century.
Clive Best therefore proceeded to do what the “consensus†failed to do, and assess carbon flows from isotopes. He concluded that over a decade or so, the atmosphere equilibrates with some much larger carbon reservoir.
One of his lines of evidence was that in the fifties, nuclear testing added a lot of carbon fourteen to the atmosphere, which, according to wikipedia, disappeared from the atmosphere over a decade or so.
Wikipedia being notoriously unreliable, I am replicating his data analysis, though we all rely on Cromer et al’s data.
Cromer et-al reported C14 measurements to 1983, and their data is on the web.
The value they report, d14C, is not the absolute level of carbon 14 in parts per million, but the change, the difference between the observed level, and the historic level before nuclear tests raised it.
I loaded their data into an open office spreadsheet, and plotted it.
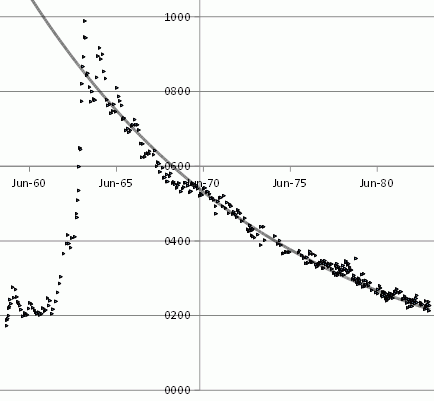
The grey line is an exponential decay, that is to say, a straight line on a logarithmic graph, of the form exp(-date/ 5330 days)
The fact that it fits the decline so well, once atmospheric nuclear testing stopped, indicates that carbon fourteen is equilibrating with a reservoir vastly larger than the atmosphere over a period of 5330 days.
And if carbon fourteen is equilibrating, so is carbon twelve and carbon thirteen, so is the carbon from coal.
So humans can no more have a significant affect on the carbon dioxide levels in the earths atmosphere, than they can raise the level of lake Michigan by spitting in it.
You need to be careful about trying to reason lunatics out of their delusions. I mean, you need to be careful not to get drawn into their delusional system in an effort to meet them halfway. That’s the origin of folie a deux – e.g. a loving wife tries to be rational about her husband’s paranoia, and ends-up indistinguishably mad.
New to me.
It’s amazing how easy it is to spot non-science with even a scrap of real science to compare it to. Hockey Team reports have so many dangling threads I can’t even call it a tissue of lies – there’s no tissue. Compared against the standard of real science, it’s indistinguishable from bare assertions. (Psychology is also a rich source of science-free papers.)
–
Wikipedia is more reliable than its reputation. You can use its own data to mostly disprove CO2 warming. Check out this picture: http://upload.wikimedia.org/wikipedia/commons/7/7c/Atmospheric_Transmission.png
The absorption band of CO2 is already saturated. Almost none of it is escaping to space anyway.
This picture is on the greenhouse gas page.
At one point, La Wik also had CO2’s greenhouse contribution at 3%. The claim has since gone up…but the fact remains that 3% sounds plausible and I got it from La Wik. (CO2 makes up 0.04% of the atmosphere. There’s simply no physical way it could capture 26% of outgoing energy.)
Let’s not get carried away with the hyperbole. There are 2 trillion tons of CO2 in the atmosphere, 50 times as much = 100 trillion tons dissolved in the ocean, 500 billion tons in the biosphere (100 billion tons estimated to be sequestered every year).
Human CO2 production is estimated at 35 billion tons per year. That’s 1/3000th the oceanic reservoir, which isn’t very much but is hardly “spitting in Lake Michigan.”
(Figures from quick Googling, corrections appreciated.)
La Wik is fun.
“Ocean acidification is the name given to the ongoing decrease in the pH of the Earth’s oceans, caused by their uptake of anthropogenic carbon dioxide from the atmosphere. Between 1751 and 1994 surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14”
Yes, significant anthro CO2 apparently started 250 years ago. Naturally, their number is negligible, and will remain that way because
“The hydration equilibrium constant at 25 °C is called Kh, which in the case of carbonic acid is [H2CO3]/[CO2] = 1.70×10-3: hence, the majority of the carbon dioxide is not converted into carbonic acid, remaining as CO2 molecules.”
Which makes sense. For every H2CO3 there must be a spare oxygen ion.
Oxygen ions are normally known as ‘basic’ or ‘alkaline.’
Secondly,
“Addition of base to an excess of carbonic acid gives bicarbonate. With excess base, carbonic acid reacts to give carbonate salts.”
A pH of 8 is basic. Which, combined with the weak CO2->acid reaction, means the ocean will end up neutralizing almost all the H2CO3 before it can do anything.
Moreover,
“With a pKa1 of 6.352, carbonic acid H2CO3 is almost 10x weaker acid than acetic acid.”
The potency of hydrogen reading doesn’t even tell the whole story. Carbonic acid is barely reactive.
A slight (but fatal) flaw with this analysis. Atmospheric CO2 doesn’t need to be sequestered, it only needs to be exchanged with non-C14 enriched carbon for the C14 in the atmosphere to go away.
There is a few hundred billion tons of biomass generation per year. That carbon comes from the atmosphere. That carbon gets added back to the atmosphere (as CO2) when the biomass decays.
There can also be exchange of CO2 with the ocean. Atmospheric CO2 goes in (removing C14), C14-free CO2 from the ocean goes out. The net exchange can be small and still lower the C14 level in the atmosphere.
What is your data source for saying that CO2 was higher 20,000 years ago?
But the reservoir the atmosphere is exchanging with has to be much bigger than the atmosphere, has to contain vastly more CO2 than the atmosphere, or else it too will shortly become as C14 enriched as the atmosphere. That the delta was converging to pre nuke test levels shows that the reservoir was big enough that exchanging with the atmosphere failed to significantly enrich it with C14. Thus, over a time period of around eleven years, atmospheric CO2 exchanges with a reservoir much bigger than the atmosphere – probably the ocean, or a substantial part of the ocean.
Humans are far too puny, frail, and insignificant to make much difference to total CO2 levels in the atmosphere and a substantial part of the ocean or something as big as the ocean.
In the 20 years of the graph, CO2 in the atmosphere went from ~320 ppm to 350 ppm. Those are actual measurements.
Your inference that CO2 is not increasing because it is coupled to a CO2 sink is demonstrably wrong. There are actual measurements of CO2 in the atmosphere over that time frame and it did not decrease, it continued to increase.
What basis do you have for saying that humans are too puny to change the atmosphere? What caused the C14 change? It was humans that caused the C14 change.
Why do you accept a quadrupling of C14 in a few years but don’t accept a few percent increase in CO2 per year? We know how much fossil fuel is being burned, and we know that the increase in the CO2 in the atmosphere is less than the amount of the new CO2 that burning the fossil fuels that we know are being burned are adding.
Your idea that humans can’t change the atmosphere is demonstrably wrong, and wrong by by the data you are using.
I made no such inference. CO2 is rising. It has risen before, it has fallen before. It has been a lot higher than it is now, and has been a lot lower than it is now. The question is whether something as puny and insignificant as humankind is causing it.
That the effect of humans on the atmospheric C14 level is short lived implies that the effect of humans on total atmospheric carbon is short lived. For non nuclear processes, the isotopes behave almost identically, so whatever happens to C14, also happens to carbon from coal.
“There can also be exchange of CO2 with the ocean. Atmospheric CO2 goes in (removing C14), C14-free CO2 from the ocean goes out.”
That there is an exchange of CO2 with the ocean supports his point. The atmosphere and the ocean are exchanging CO2. When two bodies are exchanging something, what do you think happens when you pump a whole bunch of that thing into one of those two bodies? A part of it moves into the other body. Less than the full amount remains in the first body. They equilibrate.
So, the points that you brought up support his point. You’re saying there’s an exchange exchange of carbon. That’s what he’s saying. He’s saying more than that there’s an exchange – he’s saying that since there’s an exchange, then if you pump a whole bunch of carbon into the first body, that will introduce a disequilibrium between the two bodies and they will equilibrate by means of the exchange. You might want to dispute that point but the important thing is you didn’t dispute it. You didn’t even get to it. He’s at step B and you’re at step A, and what’s more you’re at step A and you’re making statements that fail to undermine his argument; on the contrary, they support it, because if there is an exchange, then it is reasonable to suppose that there is a process of equilibration that is going through the exchange.
Here, I’ll give you an example. Suppose you have two sealed containers full of gas. There is no exchange because they’re sealed. You want to establish an exchange. That’s all you want. What do you do? You can make a little hole in each and connect them by a tube. So now there’s an exchange. But now there is also going to be equilibration. That is, suppose you have these two containers connected by the tube and you try to increase the pressure in one of the containers by pumping gas into it. So you start pumping gas into it. What do you suppose happens? Both containers increase pressure. They equilibrate. So, all you wanted was for the containers to exchange gas, but as a result, they are equilibrating.
So, once we’ve established that two bodies are exchanging something, it often (not necessarily, but often) follows that they are equilibrating. You might want to argue that that’s not happening here – that there’s an exchange without equilibration. But that’s not what you argued. You didn’t even get to the point at which you would begin arguing that.
In fact, all you’re doing is saying what he’s saying – but less so. You’re not contradicting him at all. You’re filling out part of his argument, and the only reason you didn’t get to his conclusion is that you stopped short. Maybe you have an actual argument against his argument, but you haven’t given it.
It is my belief that atmospheric CO2 is increasing. The lag in CO2 vs rising global temps is real. However, the out-gassing CO2 from the naturally warmer oceans are occurring at the same time. In this way, the percentage of anthropogenic CO2 is about the same. So, while ppm atmospheric CO2 has gone up, the percentage has not. This implies rightfully, that if we stop all anthropogenic CO2, it will have little effect on the big picture involving global warming. The present rise in CO2 may, in fact, be entirely due to a warming period around 800-1000 years ago.
You all need to go back and read the original article by Levin. It discusses the release of 14C from nuclear power plants (with increases from atom bomb detonations in China reliably recorded) whose dispersal is influenced by fossil fuel burning and local meteorological conditions in an urban setting. It says nothing about global increases in CO2 levels and confirms other observations that radioactive contributions from nuclear testing have declined since the testing stopped.
The scientific question at that time was how much radiation is released from nuclear power plants and what effects does this release have on the environment. They weren’t trying to answer the modern question of global increases in atmospheric CO2. This latter question has been answered in the affirmative rather robustly by other scientists.
What is needed is a study of the ratio of 14C to 12C (corrected for nuclear bomb emissions) over a long period of time in order to get a handle on the anthropogenic contribution to rising CO2 levels in the atmosphere.
Oh Look! Here’s a textbook example of the discussion presented in the original blog post : http://limnology.wisc.edu/courses/zoo535/classprojects/harte1_128-137.pdf
It states in an offhand manner that fossil fuels do contribute to the change in carbon ratios. Golly gee whiz.
Science rules!
Dr. Jim
Neither paper attempts to calculate the amount of carbon that is in relatively rapid equilibrium with atmospheric carbon, though it could.
enfant atteitn penguinzophren…
[…]Anthropogenic CO2 « Jim’s Blog[…]…